A preclinical trial is an experiment in which a new drug candidate is administered to animals to evaluate the safety and efficacy of the test drug. For safety evaluation, this dose is considered non-toxic if it does not harm the offspring or cells of the pregnant animal, and the dose is increased 100 times and injected into the bloodstream of the animal. Alternatively, it is evaluated by injecting 1,000 times the dose.

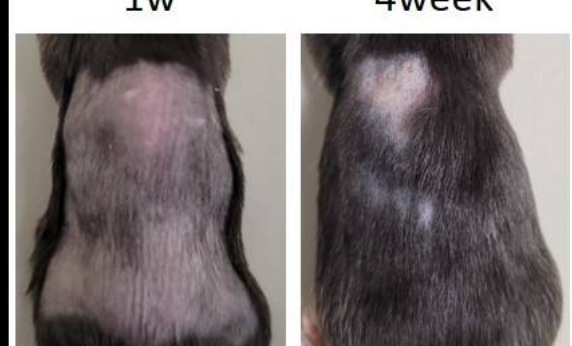
On the 22nd of last month, researchers at Panacell Biotech Co., Ltd. conducted an experiment injecting adipose-derived stem cells (ADSC) directly into the brain of a Parkinson's disease model lab rat in the company's animal laboratory.
It is believed that safety was secured in that this animal experiment used adipose-derived stem cells, which have no potential for cancer differentiation, unlike embryonic stem cells or IPS cells. In the experiment, an accurate dose was injected into the substantia niagra of the brain, which secretes dopamine, through stereotactic surgery.
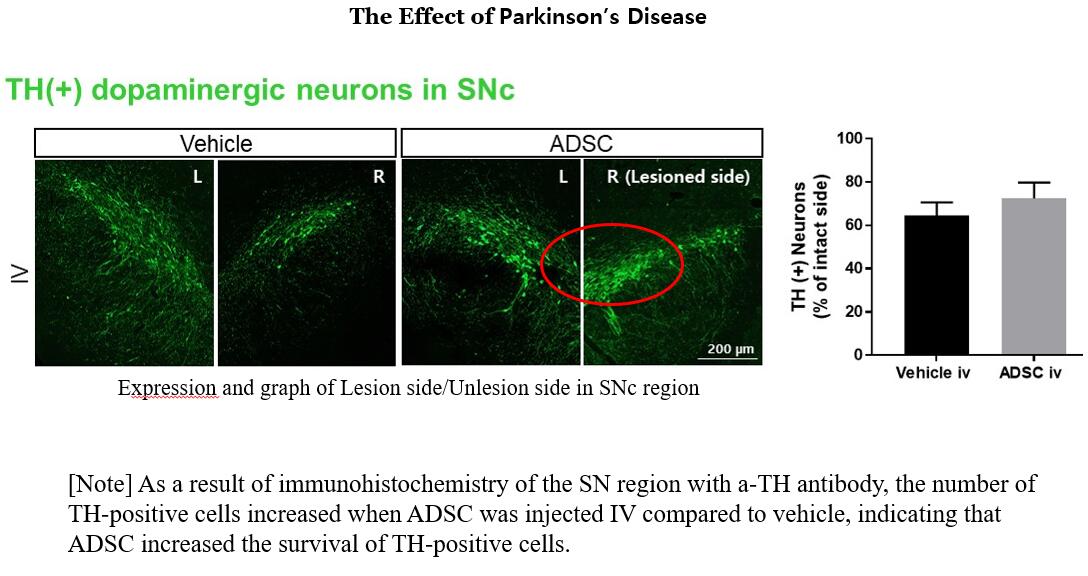
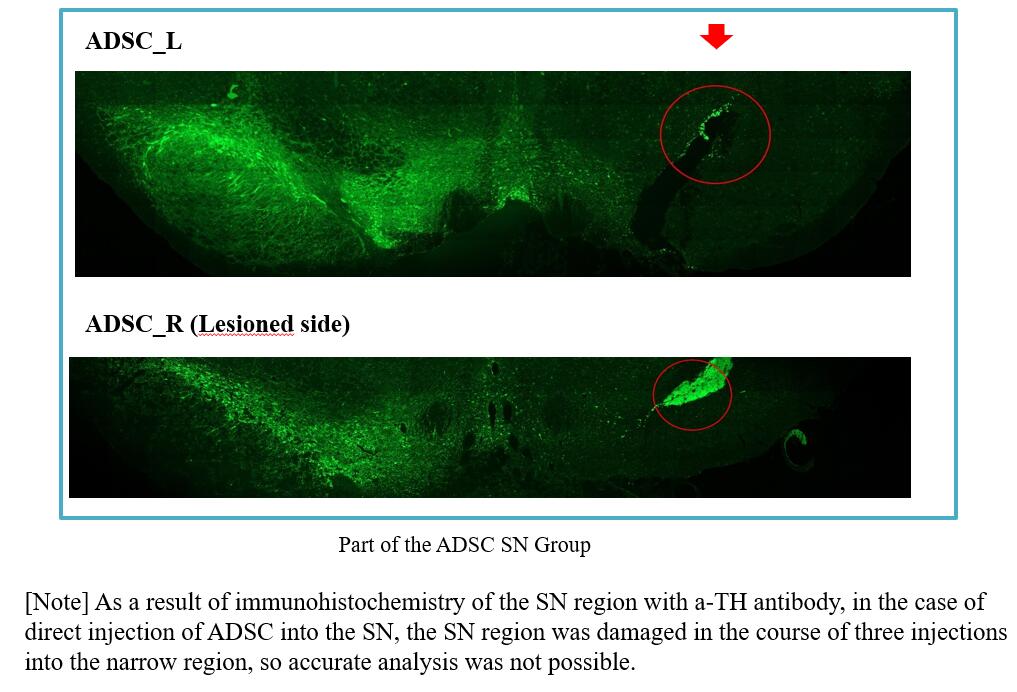
They will measure rat free locomotion characteristics, mass spectrometry of neurotransmitters and neuropeptides, immunohistochemical staining, biomarkers and beta-alkyloid levels that can diagnose early Parkinson's disease in blood. And if the toxicity and effectiveness of the drug are judged in animal tests, it will enter phase 1 and 2 clinical trials.
2023-03-15